St. Francis, Wisconsin USA
GMP intermediates, APIs and excipients (View Our Electronic Brochure Here)
(Kingchem Laboratories, Inc.)
Collapse All +Click each drop-down list below (+) to view the supporting list for each topic, or use the Expand All button above to open all drop-downs on this page at once.
MANUFACTURING CAPABILITIES
Our St. Francis, Wisconsin manufacturing plant has an experienced team that can engage at every phase of drug development, from pre-clinical through commercialization. The facility, Kingchem Laboratories Inc., offers full cGMP production for GMP intermediates, APIs and excipients, and can include the upstream integration of RSMs available through our plant in China. Click on the link below for the plant’s Chemistry Capabilities.
- Acid Chloride Formation
- Aminations
- Anhydrous HCl Handling
- Azeotropic Distillation
- Cycloadditions
- Cryogenic Crystallization
- Cryogenic Reactions
- Elemental Bromine Handling
- Esterification/Hydrolysis
- Friedel-Crafts Chemistries, Vinyl and Aromatic
- Grignard Chemistries
- Heterocycles
- Hydride Reductions
- Lewis acid catalysis
- Lithiation Chemistries
- Oxidation Chemistries
- Reformatsky Chemistries
- Reduction Chemistries
- Solid Form Manipulation
- Steam Distillation
- Transfer Hydrogenation
- Transition metal catalyzed coupling reactions
CORE TECHNOLOGIES
To see a list of the plant’s core technologies use the drop down box below. To view the chemical structures of our St. Francis Wisconsin plant’s core technologies, click here.
- Friedel-Crafts Reactions
- Grignard Reactions
- Reductions
- Oxidations
- Chiral Chemistries
- Carbocycles (Representative Compounds)
- Heterocycle Chemistry (Representative Compounds)
QUALITY ASSURANCE
The facility’s manufacturing and quality systems strictly adhere to ICH Q7 standards for Active Pharmaceutical Ingredients. ICH quality guidelines are incorporated into all aspects of our business. An effective Quality Management System led by our Quality Assurance group helps to achieve products that are consistently manufactured and controlled. KLI is registered under the U.S. FDA’s Drug Establishment Registration and is subject to routine inspections by the FDA.
- Supplier Quality / External Audits
- Internal Self Inspection Audits / Quality Improvement Initiatives
- Agency / Client Audits
- FDA cGMP, WI Division of Food Safety
- Investigation Review
- Root Cause Analysis
- Corrective Action Preventive Action
- Quality Metrics
- Data Compilation / Trending
R&D CAPABILITIES
The issues facing the industry around regulations and guidances such as Quality by Design and Q11 require that a fundamental understanding of the impact of impurities on the API quality is of utmost importance. Our analytical scientists, work hand-in-hand with process chemists, and have the tools and expertise to understand the issues related to fate-and-purge of impurities, as well as high level structural elucidation.
- R&D Laboratories
- 5 Research hoods
- 2 Walk-in hoods (to 35-L)
- 1 Distillation hood
- 5-L Hydrogenator
- Rotary Evaporators (1 to 20-L)
- Fractional Distillation to <1 torr
- Chromatographic purification
- Explorer Microwave system
- GC: 5
- HPLC: 8
- GC-MS: 1
- LC-MS: 1
- NMR: 3
- UV/VIS: 1
- Internal Kickoff Meeting
- Review Project Scope
- Assign Team
- Activity Review and Timeline Assignment
- Identify Action Items
- Raw Materials
- Qualify as Needed
- Order
- External Kickoff with Customer
- Team Teleconference
- Scope and Timeline Discussion
- Identify New Information
- Establish Communication Schedule
- Development of Process in R&D
- Informational Review
- Laboratory Familiarization Experiments
- Preliminary Risk Analysis
- DoE for QbD
- Design
- Lab Record (~Protocol)
- Execution
- Iterative Analyses
- Report
- Transfer to Manufacturing
- Best Process Description (BPD)
- Best Process Description from R&D
- Master Batch Record Compilation by Process Support
- R&D First Review, Preliminary HazOp
- MBR Adjustment to Equipment
- QA, QC, Operations Review
- Final Approval
- Process Hazard Analysis
- EHS Personnel Compile Draft
- R&D Chemist for CIM, Special Potential Hazards
- Nodal Analysis
- What If
- Risk Analysis
- Departmental Reviews
- Operator Training
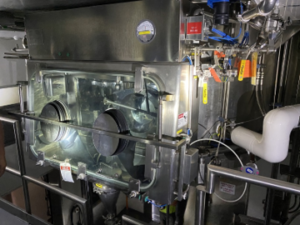
Kingchem St. Francis Wisconsin Plant
Production Bay 2

Distillation
1500-gal Hastelloy C22 Reactor with Sparging Capability

AFD

316L Stainless Steel AFD with Glove Box
AFD Glove box
KEY FEATURES
Production assets at Kingchem Laboratories range from pilot plant to full commercial scale and include a variety of materials of construction including glass-lined, stainless steel, and Hastelloy. Click on the below links for our plant’s key features and site certifications.
- cGMP: FDA registered for GMP manufacturing of pharmaceuticals (APIs, pharmaceutical intermediates, excipients).
- Experienced Staff: Key staff with 10-30 years in GMP manufacturing from CDMO to large pharma.
- Facility Footprint: 33,000 sq. ft.
- Reactor Volume Range: 35-6,300 L
- Reactor Temperature Range: -78°C to 160°C
- Reactor MOC: Stainless Steel, Glass-lined, Hastelloy
- Pressure: Up to 6 bar (90 psi)
- Distillation Capabilities: 20 L – 800 L, 20°C to 250°C (hot oil), <1 mmHg, 5-10 theoretical plates
- FDA Drug Establishment Registration
- State of WI, Dept. Safety and Prof. Services – Pharmaceutical Manufacture
- Food Processing Plant (Facility Identifier: 12671692120)
- FSSC 22000 Certification
- Kosher Certification (Chicago Rabbinical Council)
- Halal Facility Certification (Islamic Services of America)
- CTPAT certified
COMMERCIAL PRODUCTION
With each project, we set an accurate, timely schedule for our customers. Starting from either technology transfer or route selection through process R&D and scale-up, our customers enjoy our proactive and responsive style of communication and collaborative work ethic. In this way, we ensure consistent and robust production to meet your clinical and commercial needs.
- Structured packing
- Up to 25 theoretical plates
- 2x 5-gal Glass-lined
- Electric heating mantle (up to 200 °C)
- Vacuum <1mmHg
- 30-gal Stainless Steel
- Fractional (up to 160 °C)
- Vacuum <1mmHg
- 200-gal Glass-lined
- Hot oil (up to 250 °c)
- Vacuum <1mmHg
- Structured packing
- Up to 20 theoretical plates
- 50-gal gal Glass-lined
- Hot oil (up to 250 °C)
- Vacuum <1 mmHg
- 50-gal Stainless Steel
- Fractional (up to 160 °C)
- Vacuum <1 mmHg
- 200-gal Stainless Steel
- Fractional (up to 160 °C)
- Vacuum <1 mmHg
- Temperature range: 0-160 °c
- 50-gal Glass-lined
- 50-gal Stainless Steel
- 200-gal Glass-lined
- 200-gal Stainless Steel
- Temperatures as low as -78 C with certain vessels
- Pressure up to 90 psi
- 500 gal Glass-lined
- 500 gal Stainless Steel
- 200 gal Glass-lined
- 1500 gal Hastelloy
-
- 18-inch diameter monoplate SS pressure filter {holds approx. 30 kg
- 80-gal jacketed pressure SS nutsch {holds approx. 75 Kg)
- 0.6 m2 Stainless Steel
- Holds approx. 100 kg
- 1.5 m2 Hastelloy
- Holds approx. 400 kg
- 2.5 m2 Stainless Steel
- Holds approx. 600 kg
- Side Discharge
- Adjustable Discharge Rate
- Bulk Packaging
- Custom sized Packaging
- Mimic ILC Dover continuous liner system
- Filled units are placed inside a second appropriately sized outer LDPE bag
- Double bagged units are then placed inside a secondary container of the appropriate size and material of construction
- In some cases, multiple smaller units can be placed inside a large drum
- Packaging Room for sub-dividing bulk lots
- Class 8 (meets class 100,000 clean room)
- 500 gal Stainless Steel Vessel
- Reaction Temperatures to -75 ⁰C
- Jacket Temperatures to -90 ⁰C
- Closed loop thermal fluid using Syltherm XLT
- Liquid Nitrogen cooled with Heat Exchanger
API MATERIAL HANDLING
- Pharmaceutical-standard cleanability
- ISO 8 (Class 100,000) HVAC
- Class 1 Division 2 Groups C&D Electrical
- Customer viewing areas
- Single-direction flow airlocks
- Separate gowning, de-gowning
- Controlled equipment/material-handling access
- Walk-in hood, spot ventilation arm
- Flexible specific-operations PPE donning
- Primary focus:
- Final packaging
- Sampling
- Client-configuration divisions
- Allowances for future technology additions
- General Storage
- Controlled room temperature storage
- 3x Cold Storage Units
- Temp: 2-8 °c
QUALITY CONTROL
Our research chemists and quality control personnel hold educational credentials from Bachelors through Masters and PhD. The majority of experience ranges from 10-30 years in the pharmaceutical industry. Our team excels at synthesizing advanced intermediates and active ingredients and developing commercial processes to be run at Kingchem’s manufacturing facility in WI.
Kingchem has a strong QC department with dedicated specialists for developing (or transferring in from customer), optimizing, and validating analytical methods. Our instrumentation list is continuously growing and using software such as Chromeleon and Omnic 9 insures that we meet all of the 21 CFR Part 11 requirements for data integrity.
Analytical methods can be transferred in from customer or developed in-house or at one of our other locations such as Dalian or Fuxin.
- Transfer and Validation
- Approach per USP and ICH Q2 (R1)
- Protocols and Reports
- QA approved
- Client Approval Option
- Validation Protocol
- per USP and ICH Q2 (R1)
- Method Dependent
- May include linearity, accuracy, precision, sensitivity, specificity, LOD, LOQ, etc.
- Execution Time ~ 3 Weeks per Method
Support Functions
-
- Raw material release and qualification
- Routine production analyses (IPC)
- Release testing for production
- Support quality assurance
All instrumentation is regularly calibrated by original equipment manufacturers.
- Agilent HPLC 1290 Infinity II (cGMP-Compliant)
- Agilent GC FID 6890
- Agilent GC HS 7697A
- Chromeleon (21 CFR Part 11 compliant software FDA Integrity)
- Corona VEO CAD
- FTIR (Thermo Nicolet IS5)
- (with Omnic Part 11 compliant software)
- UV/Vis Spectrophotometer
- Millipore Milli-Q Water System
- NMR (PicoSpin 80)
- NMR (Nanalysis 100Pro)
- Melting Point
- Refractometer
- Metrohm KF Coulometer
- Metrohm Autotitrator
- DSC
Further equipment
- Mass Spec (R&D only)
- Sampling booth
- HEPA fan filter unit
- Slightly positive pressure to corridor
- Local exhaust for employee safety
- Logbooks
- Rotate cleaning solvents
- IQOQPQ
- Meets class 7 (class 10,000)
Production Bay 1


Cryogenic Capacity


200-Gallon GLS Reactor







